Solid gold
Posted by magazine
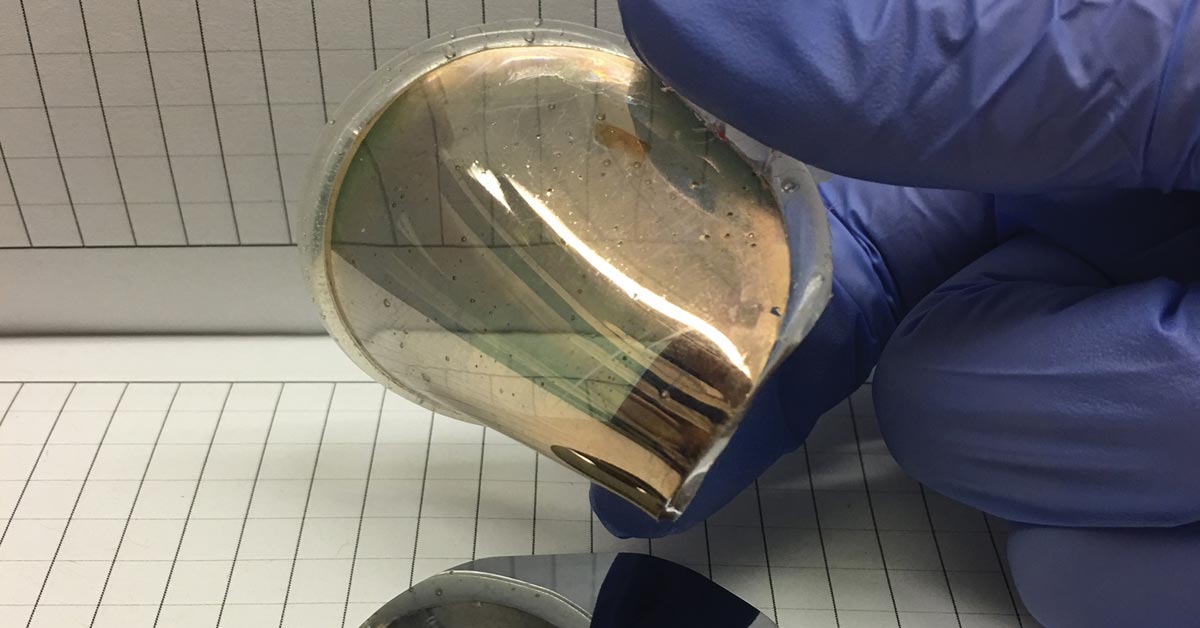
S&T researchers have developed a way to “grow” thin layers of gold on single crystal wafers of silicon, remove the gold foils, and use them as substrates on which to grow other electronic materials.
Someday, your smartphone might completely conform to your wrist, and when it does, it might be covered in pure gold, thanks to the work of Missouri S&T researchers.
In the March 2017 issue of the journal Science, S&T researchers described a new process to “grow” thin layers of gold on single crystal wafers of silicon, remove the gold foils and use them as substrates on which to grow other electronic materials. The discovery could revolutionize wearable or “flexible” technology research, greatly improving the versatility of such electronics in the future.
According to lead researcher Jay A. Switzer, the Donald L. Castleman/FCR Endowed Professor of Discovery in Chemistry, the majority of research into wearable technology has been done using polymer substrates, or substrates made up of multiple crystals. “And then they put some typically organic semiconductor on there that ends up being flexible, but you lose the order that (silicon) has,” says Switzer.
Because the polymer substrates are made up of multiple crystals, they have what are called grain boundaries, says Switzer. These grain boundaries can greatly limit the performance of an electronic device.
Most electronics on the market are made of silicon because it’s “relatively cheap, but also highly ordered,” Switzer says. “Ninety-nine point nine percent of electronics are made out of silicon, and there’s a reason — it works great. It’s a single crystal, and the atoms are perfectly aligned. But when you have a single crystal like that, typically, it’s not flexible.”
By starting with single crystal silicon and growing gold foils on it, Switzer is able to keep the high order of silicon on the foil. But because the foil is gold, it’s also highly durable, flexible and nearly transparent. Switzer and his team have peeled foils as thin as seven nanometers. (A nanometer is one billionth of a meter.)
Switzer says the challenge his research team faced was not in growing gold on the single crystal silicon, but getting it to peel off as such a thin layer of foil. Gold typically bonds very well to silicon.
“So we came up with this trick where we could photo-electrochemically oxidize the silicon,” Switzer says. “And the gold just slides off.”
Photoelectrochemical oxidation is the process by which light enables a semiconductor material, in this case silicon, to promote a catalytic oxidation reaction.
Switzer says thousands of gold foils — or foils of any number of other metals — can be made from a single crystal wafer of silicon. ο
“So we came up with this trick where we could photo-electrochemically oxidize the silicon, And the gold just slides off.”
S&T researchers have developed a way to “grow” thin layers of gold on single crystal wafers of silicon, remove the gold foils, and use them as substrates on which to grow other electronic materials.