Your own…personal…fuel cell
Posted by Mary Helen Stoltz
By 2015, drivers may be less concerned about gas mileage than about hydrogen storage. By 2030, the United States’ dependency on foreign oil to power our cars and trucks could be a thing of the past.
A few years later, homeowners might be able to drop off the grid, generating their own power from in-house fuel cells and leaving behind nothing but clean, potable water.
Yangchuan Xing, assistant professor of chemical and biological engineering, is working to bring these possibilities into reality using polymer electrolyte membrane (or PEM) fuel cells.
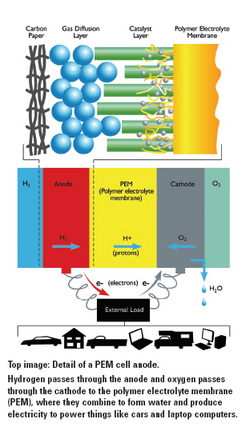
In a PEM fuel cell, electrons are conducted across two electrodes with a polymer membrane sandwiched between them. The anode is fueled with hydrogen to produce protons and electrons. The polymer membrane conducts the protons through to the cathode, where they recombine with electrons and oxygen to form water, the only byproduct of a PEM fuel cell. The process creates the electricity to power devices from laptop computers to cars to homes.
“It’s a very environmentally friendly process,” Xing says.
PEM fuel cells can be used in a residential setting, providing power to homes through either stationary in-house fuel cells that operate like big batteries, or through larger power station-sized cells that could power an entire city. But the Department of Energy’s main focus is on their uses in the automotive industry, Xing says.
“The DOE is focused on developing safe, inexpensive PEM fuel cells for use in vehicles in the hope of developing new technologies that would lower the cost,” he says. “Ford, Chrysler and General Motors, as well as the Japanese car companies, have demonstrated fuel cell-powered cars.” However, with a sticker price of more than $100,000, they are still too expensive for the average consumer.
Xing is working to help reduce that cost by developing a more efficient PEM fuel cell using a new material called carbon nanotubes. The traditional material used, carbon black, corrodes easily in fuel cells. The different structure of Xing’s carbon nanotubes causes them to corrode much slower and improves their durability.
“We like the fuel cells to run for 5,000 hours and beyond,” Xing says. “Using carbon black, you can’t get there without corrosion. Our research shows that carbon nanotubes stabilize.”
Carbon nanotubes are still expensive, but they extend the life of the fuel cell, so there is a tradeoff, Xing explains.
Green machine
Powering cars and trucks with a fuel whose only byproduct is clean water would go a long way toward reducing air pollution. It would be a major advantage to the trucking industry, which produces a great deal of harmful pollutants like greenhouse gasses, Xing says. “If you equip these trucks with hydrogen fuel cells, the only thing released into the environment is water that is clean enough to drink.”
Hydrogen used to power PEM fuel cells in automobiles can be stored in one of three ways. The hydrogen can be stored in tanks in either liquid or compressed gas form. But the preferred method is to absorb the hydrogen onto a powder. This solid state storage is considered the safest method. Xing is working to improve this process using a nanocomposite powder made up of carbon nanotubes with metal nanoparticles, a project funded by General Motors.
“If you have a tank of hydrogen on your car, it’s like a bomb,” Xing explains. Solid state hydrogen is much less volatile. “When you’re ready to use solid state hydrogen, heat it a little or reduce the pressure a little and the hydrogen will desorb from the powder and move into the fuel cell.”
Not your father’s hydrogen fuel cells
PEM fuel cells have several advantages over their older solid oxide relatives. In addition to having a lower operating temperature, they also have a faster response time, all of which make them more appealing for use in automobiles.
Solid oxide fuel cells run at nearly 1,472 degrees Fahrenheit and require a cooling system to operate. The cooling system increases the power load of the car and consumes more fuel. PEM fuel cells, however, run at only 176 degrees Fahrenheit and require no cooling system. They can be started at much lower temperatures, allowing them to be used in vehicles even in cold winter climates.
“In a car, you want to start the fuel cell and get power right away,” Xing says. “A PEM fuel cell can be ‘cold started’ at room temperature and be ready to operate the vehicle in just a minute or two.” Because solid oxide cells have to reach such a high operating temperature, it can take 10 minutes or more before they’re ready to run.
On a smaller scale
In his latest project, a three-year $310,000 study funded by the National Science Foundation, Xing is developing new technology to increase the power of smaller micro PEM fuel cells that can be used to power portable electronic devices like cell phones and laptop computers.
Micro PEM fuel cells are fueled by liquid methanol, instead of hydrogen, making them safer and easier to handle. Each methanol cartridge, depending on its size, can provide power for up to eight hours. Current lithium batteries last only two to three hours without recharging.
These are predicted to be the first PEM fuel cells to penetrate the market, Xing says, because they’re less cost prohibitive in comparison with the lithium batteries that are currently being used, which are also quite expensive. “Some Japanese companies are already marketing electronics equipment with fuel cells,” Xing says.